Your email address will not be published. There are some differences between clean area aseptic area and sterile area which are listed.

Designing Of Aseptic Area Part 1 Unit 4 Microbiology B Pharma 3rd Sem Carewell Pharma Youtube
Aseptic area is an extremely important work place for sterile preparation.

. It was also great to work as a team and to discuss and rationalise the decisions that were made. Area grades as specified in section 1751 1753 must be selected by the manufacturer on the basis of validation runs eg sterile media fills WHO TRS No. Aseptic processes should meet grade A with unidirectional air flow.
Isolates from the controlled environment where aseptic processing is to be conducted may also be used. The aseptic unit is designed to carry out each stage of production separately. US standards define the controlled area as the areas where Non-sterilized products are prepared.
Aseptic processes should minimize the exposure of sterile articles to the potential hazards of contamination during the manufacturing operation. Production of sterile products should be carried out in a clean environment with a limit for the environmental quality of. To include as.
Figure 1 Comparative Particle Sizes. Over and above the design process is a critical area in the pharmaceutical industry. No differences to Class A.
Sterilized operation critical Area aseptic application Controlled Areas. See Figure 1 below for comparative sizes of particulate. 823 Annex 1 1992 5 5.
There is no specific general cleanroom classification requirement for all non-sterile drugs However in the direct Aseptic area exposed sterile product the class must be 100 which we will discuss later. A Lot to Learn. Equipment must be of the simplest design possible for the operation being performed.
What does this mean. This includes areas where compounds are compounded and where components in-process materials drug products and contact surfaces of equipment containers and. Designing of Aseptic Area Sources of Contamination in Aseptic Area.
Leave a Reply Cancel reply. Non viable air count 3520 05 μ 29 5 μ m 3 at rest and 352 000 05 μ 2900 5 μ m 3 in operation. All content in this area was uploaded by Tim Sandle on Jul 31 2016.
Grade C Pharmacy Aseptic Suite Floor area. Sweeps contaminants away from work surface area. Facility Design Cleanroom facilities are designed to go from lower class to higher class less clean to more clean Each subsequent clean space requires additional controls to prevent ingress of undesired items Goal is to make it more difficult for contamination to occur as you get cleaner Minimize critical area space.
Following the aseptic processing of the medium the filled containers are incubated at 225 25 or at 325 25. 270 m 2 Contract value. The use of isolators for aseptic processing is also discussed.
It is therefore essential that workers are well trained and motivated and familiar with the task in hand. Design and Construction Features. In this video here is explanation about specifications factors consideration for.
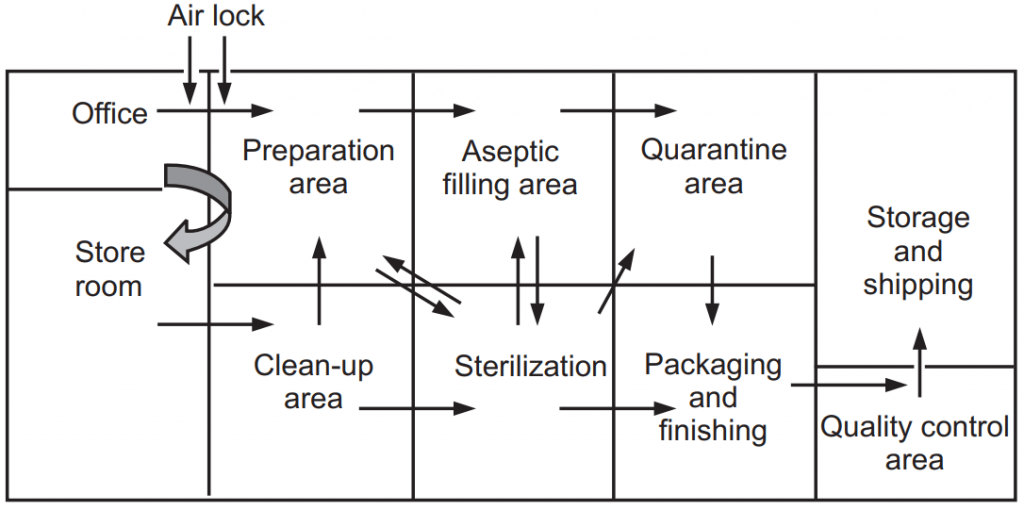
Design of aseptic areatesting of clean and aseptic rooms. Air velocity 045 ms 20 at filter face. The production area is normally divided into the clean-up area the compounding area the aseptic area the quarantine area and the packaging area.
All media filled containers. Under certain circumstances turbulent airflow may be justified in a closed isolator when proven to have no negative impact on the product. Designing of Aseptic Area.
Speed accuracy and economy of movement are essential features of good aseptic technique. Pharmaceutical Microbiology 3T5 By. The production area for sterile products is normally divided into Clean up area.
Issues that you should notice include well-designed processes in limiting the duration of exposure of sterile products good environment. Appropriate devices to monitor and display these conditions inside the aseptic area may be installed. Aseptic and less critical activities.
Air flow Away from critical points. The temperature should be maintained at 210C 30 C inside the aseptic area all the time with corresponding relative humidity between 20 to 60 though the ideal RH is considered to be 55. July 11 2021 by Sujay Mistry.
Aseptic manipulations must be carried out in the grade A air of a laminar flow cabinet or isolator. Enbloc were selected to design and build of a major new facility direct for the NHS Trust. The aseptic area is the area where strict control measures are adopted to avoid contamination of the preparations or any transfer of microorganisms.
Flow diagram of aseptic area Floors walls and ceilings All clean surfaces including the floor walls and ceilings must be smooth easy to clean disinfected and be constructed to minimize microbial and particulate contamination. Design features No drains sinks. The unit should also ensure a safe and organised workflow so that the need for personnel to move around the clean rooms is minimised.
Hence proper care should be taken while designing the aseptic area. The project was a new Pharmacy Aseptic Unit which will manufacture Chemotherapy Total Parenteral Nutrition TPN Antibiotics and various intravenous drugs and. Published by Suman Kumar Mekap on May 5 2019 May 5 2019.
Aseptic Process Area Design. Design level or worker comfort level. Definition Aseptic area surrounding the Class A filling zone.
This assignment was a great opportunity to go deep into the design considerations of a biopharmaceutical facility especially of an aseptic processing area. School of Studies in Pharmaceutical Sciences Jiwaji University Gwalior. The design of the RABS and open isolators should ensure a positive airflow from the critical zones to the surroundingareas.
Particulate monitoring during aseptic product filling and APS consists of continuous monitoring for particulates in the 05 μm and 50 μm ranges using a particle sampler attached to an isokinetic probe located near to the point of fill in the Grade A area. Design of aseptic areatesting of clean and aseptic rooms. Qualification cleanroom design process design quality control environmental monitoring and review of production records.
Must be produced from sterile starting materials in an aseptic way. Easily cleanable floors wall and. Required fields are marked.
An aseptic compounding laboratory previously occurring during the third-year spring semester was added to.
Designing Of Aseptic Area Solution Pharmacy

Design Of Aseptic Area Testing Of Clean And Aseptic Rooms Labmonk

Designing Of Aseptic Area Youtube



0 comments
Post a Comment